Antibody Detection
DETECTION OF ANTIBODIES TO SARS-COV-2, THE VIRUS THAT CAUSES COVID-19
April 13, 2021 UPDATE
This review was prepared with significant input from the following contributors:
- Jeffrey P. Henderson, MD, PhD, Associate Professor of Medicine and Molecular Biology, Division of Infectious Diseases, Center for Women's Infectious Diseases Research, Washington University School of Medicine, St. Louis, MO
- Christopher W. Farnsworth, PhD, Assistant Professor of Pathology & Immunology, Washington University School of Medicine, St. Louis, MO
- Ernst J. Schaefer, MD, Professor of Medicine, Tufts University School of Medicine, Boston, MA, Chief Medical Officer & Laboratory Director, Boston Heart Diagnostics, Framingham, MA
- Elitza S. Theel, PhD, D(ABMM), Director of Infectious Diseases Serology Laboratory, Co-Director of Vector-Borne Diseases Laboratory Service Line, Associate Professor, Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN
Background
After infection with a pathogen, such as a virus, bacteria, fungus or parasite, the human immune system recognizes specific components (called antigens) from that pathogen as foreign. The immune system can then respond to this antigen by making antibodies, which are small proteins that recognize and bind to these foreign viral antigens, and through various mechanisms prevent the pathogen from infecting new host cells. There are different antibody classes, which have different development time courses. In general, IgM-class antibody levels rise in the first 3-7 days after viral infection, followed shortly thereafter by the development of IgG-class antibodies. Typically, IgM antibodies rapidly decline over the next 2-3 months, whereas IgG-class antibodies remain elevated for prolonged periods of time, which depending on the pathogen, can be months to years.
For SARS-CoV-2, the virus that causes COVID-19, while some individuals may develop antibodies to the virus during the first week after developing symptoms, most patients become antibody positive 2 to 3 weeks after the onset of symptoms. In most symptomatic patients, IgM and IgG class antibodies appear at about the same time (see Figure), but how long antibodies to the SARS-CoV-2 virus last is still unclear, as we discuss below.
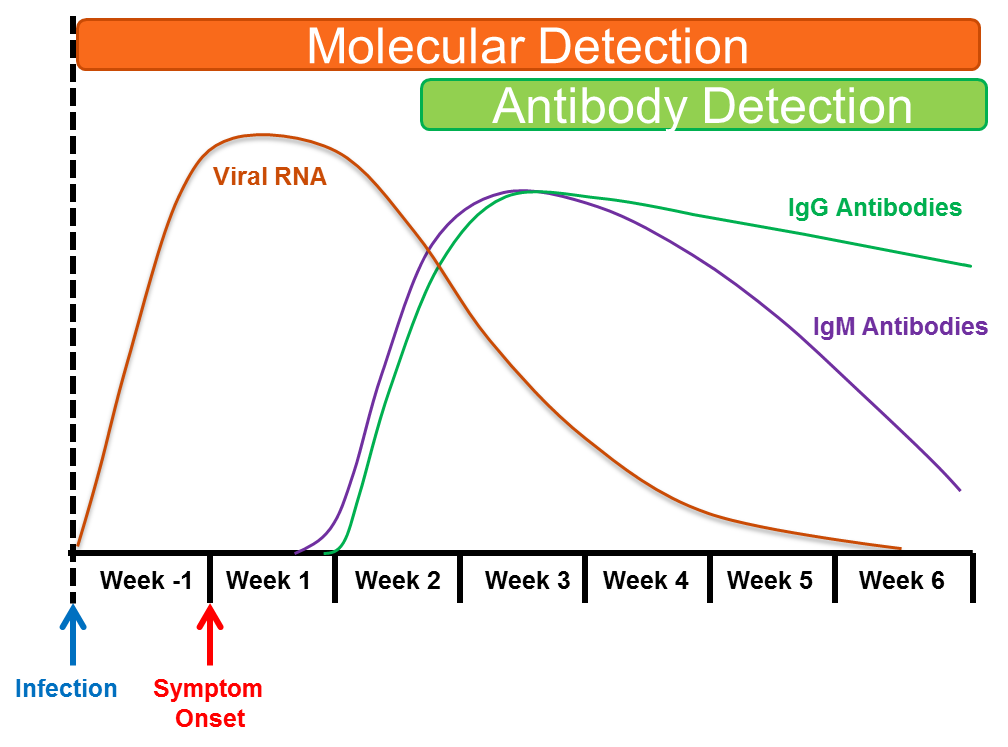
Tests that detect antibodies to particular pathogens can be useful to document past infection and to indicate potential immunity against re-infection. These tests are referred to as serologic tests, and the transition from antibody-negative (before infection) to antibody-positive (after infection) is referred to as seroconversion.
It is important to understand that serological or antibody tests do not directly detect the virus or pathogen itself. Rather, serologic tests are an indirect way to determine whether your body’s immune system has previously encountered the virus. In general, a serologic test cannot clearly reveal when an individual was infected – the serologic test result can be ‘positive’ whether infection was 3 months ago or 3 weeks ago.
To detect a current infection with the SARS-CoV-2 virus requires a molecular test to detect viral genetic material or other antigen, most commonly using reverse transcriptase PCR [RT-PCR]) (see virology on this website).
Over 70 different serologic tests have received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA) to be eligible for use in patients. These tests vary significantly in design and format. Briefly, differences can be grouped in the following categories:
1) ASSAY FORMAT
- Lateral Flow Assay (LFAs) These tests are typically rapid, able to be completed in 10-20 minutes and are may be used at the point-of-care. Independent assessment of the specificity and sensitivity of these assays has indicated lower values than obtained with laboratory-based immunoassays.
- Enzyme-linked immunosorbent assays (ELISAs) or chemiluminescent immunoassays (CIAs). These assays are typically performed in clinical laboratories and require instrumentation to perform. They are high-throughput assays, able to complete 1000s of tests or more per day and are usually significantly more accurate than LFAs. Some of these tests do quantify the actual amount of antibody present, while other assays are only qualitative providing a positive or negative result.
2) TARGET ANTIBODY. These assays may detect IgM- or IgG-class antibodies individually or together on one test. Alternatively, the serologic assay may be designed to detect ‘total’ antibodies, meaning all antibody classes, without differentiating or indicated which specific class is detected.
3) TARGET SARS-COV-2 ANTIGEN. Serologic assays may be designed to detect antibodies against different parts (or antigens) of the SARS-CoV-2 virus. These SARS-CoV-2 antigens generally include one or both of the proteins described below:
- Spike protein. The spike protein is the major protein on the surface of SARS-CoV-2 that is responsible for binding to human cells.
- Nucleocapsid protein. This protein is found on the inside of the virus and surrounds the genetic material. It is the most abundant protein in the virus.
4) ASSAY RESULTS.
- Qualitative Tests. The majority of serologic tests for SARS-CoV-2 antibodies are qualitative, meaning that they provide results as only ‘positive’, ‘negative’ or ‘indeterminate/equivocal’. Although these serologic tests still result in a number (e.g., index value, signal-to-cut-off [S/Co] ratio, etc.), these numbers do not indicate the actual antibody level and should not be reported.
- Semi-Quantitative or Quantitative Tests. Increasingly, serologic test manufacturers are developing methods that provide antibody levels in the blood sample.
- For these tests, the amount of antibody may be reported as titers, arbitrary units per milliliter (AU/mL) or unit per milliliter (U/mL), among others.
- Importantly, although the World Health Organization has released an international standard for SARS-CoV-2 IgG, most serologic assays have yet to be standardized against it. As a result, the values generated by one kind of serologic test cannot be compared to another.
- The advantage of quantitative assays is that they may provide a guide to which plasma donors may provide high levels of IgG antibodies for hospitalized recipients. In general high levels of IgG antibodies correlate with high levels of neutralizing antibodies.
A number of manufacturers have now developed qualitative assays for neutralizing antibody detection, which have been validated versus cell based assays that document viral killing.
5) ACCURACY
The accuracy of these serologic tests that have been approved by the FDA for emergency use have been evaluated quite extensively in the medical literature. The FDA website can provide more information on these assays. It is important to understand that no serologic assay has perfect accuracy, meaning that false positive (meaning that the test says you are positive but you really negative) and false negative results (meaning the test tells you are negative but you are really positive). The risk of these occurring depends on multiple factors, as outlined below.
- Possible Causes of False Negative Results:
- The sample was collected too soon following infection, prior to the development of enough antibodies to be detected by the test.
- The sample was collected too long after infection. Although the duration of antibodies against SARS-CoV-2 continues to be studied, emerging data suggest that the longevity of detectable antibodies is dependent on multiple factors, including:
- Severity of initial infection, with asymptomatic or mildly ill patients developing lower antibody ‘levels’ as compared to severely ill patients,
- The patient immunostatus, or
- The type of serologic assay used
- Possible Causes of False Positive Results
- The SARS-CoV-2 serologic test detected antibodies to another closely related pathogen such as another coronavirus that causes colds, not COVID-19 disease. This is called cross-reactivity or non-specific binding. This has generally not been found to be a significant problem for SARS-CoV-2 antibody testing.
- Low Positive Predictive Value (PPV) of the serologic test. In general, testing of individuals who reside in areas where the prevalence of SARS-CoV-2 infection is low or who have a very low risk of prior infection, is linked to a large chance that any positive antibody result is actually a false positive. This is because even a small false positive becomes more important when the true positive rate (which depends on the prevalence of the infection in the community being tested) is very small.
Frequently Asked Questions
QUESTION 1. If I am antibody positive, does that mean that I am protected from re-infection with SARS-CoV-2?
ANSWER: Recent studies suggest that previously naturally infected patients are at significantly lower risk for SARS-CoV-2 reinfection for at least 8 months. Similarly, data continue to emerge that vaccinated individuals are also as significantly lower risk of severe disease compared to unvaccinated individuals, for at least 6 months. However, it remains unclear how long antibodies remain in blood after infection or for how long they are protective. Therefore, individuals with positive antibody results for SARS-CoV-2 should continue to wear masks, wash hands and physically distance as recommended by their state and local public health departments.
QUESTION 2. My SARS-CoV-2 antibody test result provided me a number. What does that number mean?
ANSWER. Currently, most serologic assays for SARS-CoV-2 are qualitative, meaning that only a ‘positive’, ‘negative’ or ‘indeterminate/equivocal’ result should be provided. The numerical values associated with qualitative serologic tests do not mean anything and should not be used to make any clinical or personal decisions.
If the serologic test used was semi-quantitative or quantitative, the reported value indicates a general level of antibodies in your blood. However, exactly what level of antibodies are necessary for protection against re-infection and how long those last, remain undetermined. Contact your healthcare provider to determine whether the serologic test that was used is qualitative or quantitative in nature.
QUESTION 3. If I am antibody positive for SARS-CoV-2, can I donate plasma for the Convalescent Plasma Therapy program?
ANSWER. Yes. After you donate, your blood will be tested again for antibodies to determine what level of antibodies are present. This is required by the FDA to determine whether your plasma contains enough antibodies to help COVID-19 patients. Remember that you must have been asymptomatic for at least 14 days before donating plasma.
QUESTION 4. What are neutralizing antibodies?
ANSWER: Neutralizing antibodies are antibodies that can bind to a pathogen such as SARS-CoV-2 and “neutralize” or block its ability to infect cells. While this may be one important way that antibodies protect against viral infections, antibodies themselves and the broader immune system can provide protection in other ways as well.
- At this time, the degree to which neutralizing antibodies provide protection is unknown and the concentration of neutralizing antibodies required to confer protection is unknown.
- There is quite strong evidence that neutralizing antibodies correlate quite closely with IgG levels in serum.
- Measurement of neutralizing antibodies requires special laboratories called BSL-3 facilities that are able to handle live SARS-CoV-2. A number of manufacturers have now developed immunoassays for neutralizing antibody, some of which have FDA EUA, that have been validated versus cell- based assays and these do not require such specialized facilities.
- Researchers have created so called pseudovirus assays, which use a portion of SARS-CoV-2 that allows an unrelated virus to gain entry to cells in a laboratory. These assays measure the amount of antibody necessary to protect cells from infection. While pseudovirus assays have higher throughput and are scalable, none are currently approved for use with clinical specimens.
QUESTION 5. If I have a positive COVID-19 antibody result, do I have neutralizing antibodies?
ANSWER. Most individuals infected with SARS-CoV-2 do develop some level of neutralizing antibodies, and we believe that there is a correlation between neutralizing antibodies and the findings of most currently available serologic assays. However, there is no commercially available assay that directly assesses the presence of neutralizing antibodies, although these are under development now.
COVID-19 Serology Tests Performance Measures
FDA EUA authorized COVID-19 Serology Tests TableReferences
-
- Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infectious Diseases. 2020;7(3).
- Stadlbauer D, Amanat F, Chromikova V et al: SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr Protoc Microbiol. 2020 Jun;57(1):e100. doi: 10.1002/cpmc.100.
- Okba NMA, Müller MA, Li W: Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease 2019 Patients. Emerg Infect Dis. 2020 Apr 8;26(7). doi: 10.3201/eid2607.200841. [Epub ahead of print].
- Guo L, Ren L, Yang S: Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020 Mar 21. pii: ciaa310. doi: 10.1093/cid/ciaa310. [Epub ahead of print].
- National COVID Testing Scientific Advisory Panel. Evaluation of antibody testing for SARS-CoV-2 using ELISA and lateral flow immunoassays medRxiv preprint doi: https://doi.org/10.1101/2020.04.15.20066407
- Deeks JJ, Dinnes J, Takwoingi Y etal. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No.:CD013652. Doi:10.1002/14651858.CD013652
- CBER. Investigational COVID-19 Convalescent Plasma - Emergency INDs. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ideprocess-cber/investigational-covid-19-convalescent-plasma-emergency-inds.
- Woelfl R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID-19. Nature. 2020;581(7809):465-469. Doi:10.1038/s41586-020-2196-x
- Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. doi:10.1001/jamainternmed.2020.4130 Published online July 21, 2020.
- Dulipsingh L, Ibrahim D, Schaefer EJ at al. SARS-CoV-2 serology and virology trends in donors and recipients of convalescent plasma. Transfus Apher Sci. 2020 Aug 25:102922.
- Maruato AE, Fontes-Garfias CR, Ren P et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nature Communications 2020;11:4059 | https://doi.org/10.1038/s41467-020-17892-0.

